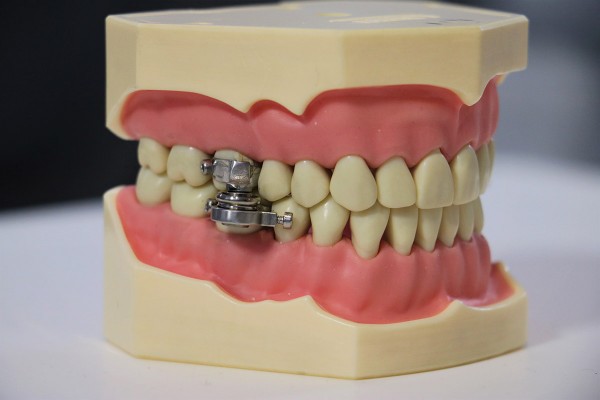
The Otago-led research team behind the ‘DentalSlim Diet Control Device’, a magnetised jaw clamp that prevents people from eating solid food, claimed in their ethics committee application that their product would have “no real ethical issues” and presents “no significant risks” — claims accepted by the Ministry of Health’s Health and Disability Ethics Committee (HDEC).
The ethics committee application form was made publicly available online after an Official Information Act request to the University of Otago was filed by Emma Joyce on the website FYI.org.nz. The University redacted the names of everyone involved in the approval and research of the project, apart from lead investigator Professor Paul Brunton, saying it was “necessary to protect employees of the University from … improper pressure or harassment.”
Prior to beginning the application, the project was approved by a Head of Department in the Faculty of Dentistry, who noted that “I support this study,” and by a second University staff member, who called this “an interesting and potentially very useful study.”
Asked to “provide a brief summary of the main ethical issues you believe your study may raise,” the researchers responded that “There are no real ethical issues here,” noting similar treatments have been provided before, “in the form of jaw wiring”. The use of jaw wiring as a treatment for obesity was used until the 1980s, with researchers frequently finding weight gain once the device was removed, as well as significant decreases in quality of life during the period of treatment. Clinical psychologist Louise Adams told Critic Te Arohi: “Re-booting an invasive ‘treatment’ without any consideration or discussion of the harm done in the past is incredibly irresponsible.”
The researchers further asserted that this study carried “no risks of stigmatisation of individuals or population groups,” indicating that very few of them had ever tried to live life with their jaws magneted shut. In their study, there were increases in the proportion of participants who reported feeling “tense,” “embarrassed,” and “self-conscious because of the device” — the researchers claimed this was mainly due to its size, and hoped to conduct future studies using a smaller version with “better aesthetics”.
Dismissing potential risks and embarrassment in this way, says Louise, “reveals just how widespread weight bias has legitimised ever more invasive ‘interventions’ on people’s bodies. The ‘problem’ of an obese body is assumed to be so bad, that potentially very dangerous, even fatal, (choking events) consequences were not even mentioned …. It is incredible that the authors do not consider either the existing status of larger people as highly stigmatised and do not consider their extreme device to contribute further to this.”
Additionally, researchers’ response to a question asking how the study would “contribute to reducing inequalities in health outcomes between different populations, and particularly between Māori, Pacific peoples and other New Zealanders” was also copy-pasted into a section about “whether and how the study may benefit Māori”. As a result, in a question focusing on Māori, obesity rates among Pacific Islander adults were cited, in addition to those in people with “less education and lower socio-economic status, especially women”.
This ethics application form was sent to the HDEC’s Northern B Ethics Committee for review, which included a meeting with Professor Brunton by teleconference. The publicly available minutes from that meeting show only minor changes were requested by the Committee. Amendments to some questions in the device questionnaire, the addition of a picture of the device to the participants’ sign-up sheet and the clarification to participants that the costs of transport and liquid food would be paid for. There was no mention of any other potential ethical issues or risks.
Asked about whether the ethical approval process was rigorous enough, a Ministry of Health spokesperson responded: “The HDECs follow and apply very robust ethical standards, (this study) had been peer reviewed by appropriate experts, and underwent consultation with Māori. All participants gave informed consent to be in the study and could withdraw at any time prior to or during the planned study period. There were no reported adverse events in relation to participants. The committee reviews all study documentation which includes details on the risks involved in studies, and inclusion exclusion criteria, to minimise risks to participants.
The University of Otago told Critic Te Arohi that they were not directly involved in the ethics approval process, and could not directly comment on that. However, Professor Richard Blaikie, Deputy Vice-Chancellor, Research and Enterprise, said: “The University has confidence in the HDEC process for identifying ethical issues, which principally focus on research participants and their willingness to participate with clear understanding of what is involved and the potential benefits of the research.”
While the DentalSlim seems like a silly experimental device that would be easy to dismiss, Louise says it has already caused serious harm: “Larger bodied clients of mine have reported feeling extremely traumatized … it makes them feel ‘hunted’. It sends a message to larger people that their basic human right to eat food is not respected, and that dystopian torture devices are seen as ‘viable’ by researchers and universities.”